Choose the Most Stable Conformation of Trans-1 4-diethylcyclohexane
This means that the cis isomer 13-dimethylcyclohexane is more stable than the trans isomer. Cis 1 4 Dimethylcyclohexane Chair - 14 images - las conformaciones del is mero trans sonid nticas solved 17 which of the following chair conformations rep 1 3 dimethylcyclohexane structural formula solved for cis 1 3 dimethylcyclohexane which structures.
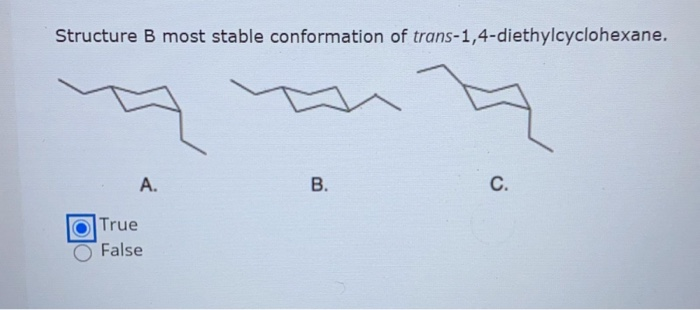
Solved Structure B Most Stable Conformation Of Chegg Com
What is the difference in energy between the two chair conformations of trans-14-dimethylcyclohexane.
. 3 Chemical and Physical Properties Expand this section. Which of the following has two equatorial alkyl substituents in its most stable conformation. B the methyl group is axial and the tert-butyl group is equatorial in a chair conformation.
In each of the boxes below draw in methyl Me groups in the appropriate positions. 2 Names and Identifiers Expand this section. Ethylcyclohexane Explain with the aid of conformational structures why cis-13-dimethylcyclohexane is more stable than trans-13-dimethylcyclohexane whereas the reverse order of stability is observed for the 12 and 14.
100 6 ratings Transcribed image text. Experts are tested by Chegg as specialists in their subject area. Cycloalkanes And Their Stereochemistry.
Alkanes And Their Stereochemistry 4 Organic Compounds. Identify the least stable conformation of trans-14-diethylcyclohexane. A the tert-butyl group is axial and the methyl group is equatorial in a chair conformation.
The more stable chair conformation of trans-12-dimethylcyclohexane has the two methyl groups in the equatorial position. Acids And Bases 3 Organic Compounds. Correct option is A Usually for derivatives of cycloalkanes the chair conformation with bulky substituents placed at the equatorial positions is more stable as 13 diaxial interactions of bulky groups with hydrogen atoms are avoided.
View Test Prep - chem from BIO 321 at Hogwarts School of Witchcraft Wizardry. 4 points CH3 Least stable chair Most stable chair CH3G Ð36 kcalmol Question 3 is continued on the. In chair conformation of cyclohexane we have two position in the conformer.
In the most stable conformation of trans-14-dimethylcyclohexane what positions do the methyl groups. Correct option is D The stable conformers of trans-14-dimethyl cyclohexane is 1-equatorial-4-equatorial form. B trans-14-Dimethylcyclohexane shown below also exists in two different chair conformations one of which is 36 kcalmol more stable than the other.
Draw the two chair conformers for each of the following and indicate which conformer is more stable. Solve any question of Organic Chemistry - Some Basic Principles and. 14-diequatorial conformation is most stable as the steric interactions are minimum.
A handy way of determining the substitution alternatives is to use the. Here in case of 1-chloro-4-methylcyclohexane CH 3. The first step in drawing the most stable conformation of cyclohexane is to determine based on whether the substituents are cis or trans to one another and based on where theyre located on the ring what the choices of axial and equatorial positions are for the substituents.
Contrary to the case of methylcyclohexane which has no interactions in the chair conformation having an equatorial methyl group the diequatorial conformer of trans-12-dimethylcyclohexane has a gauche butane interaction red and blue. When a substitutent is present at axial position the conformer will be less stable because it has 1 3 diaxial interaction which is a steric interaction of axial group. If one methyl group is in the lower-energy equatorial position then the cis compound with both methyl groups on the same side of the ring can be made only by placing the second methyl group in the higher-energy axial position.
Identify the compound where the groups are axial and equatorial. And Cl are when placed at equatorial position in chair. The most stable conformation of cis-1-tert-butyl-2-methylcyclohexane is the one which.
Cis and trans formspossible isomers of cis - and trans -14-dimethylcyclohexane. This will increase the. A 11-dimethylcyclohexane B cis-12-dimethylcyclohexane C cis-13-diethylcyclohexane D cis-14-diethylcyclohexane E trans-13-diethylcyclohexane.
We review their content and use your feedback to keep the quality high. Trans-12-dimethylcyclohexane is a cycloalkane. Ring Strain 44 Conformations Of Cycloalkanes 45 Conformations Of Cyclohexane 46 Axial And Equatorial Bonds In Cyclohexane 47 Conformations Of Monosubstituted Cyclohexanes 48.
In the lefthand structure the two blue methyl groups are both axial on opposite faces of the ring. 1 Structures Expand this section. 1 Structure And Bonding 2 Polar Covalent Bonds.
Cycloalkanes And Their Stereochemistry 5 Stereochemistry At Tetrahedral Centers 6 An. 14-diaxial conformation is least stable as the steric interactions are maximum. Each side of the ring is effectively an axial methyl cyclohexane.
Draw the formula for the preferred conformation of a. Therefore the total energy is 18 18 36 kcalmol. As can be seen in the structures above cis -13-dimethylcyclohexanes most stable conformer has both methyl groups equatorial while trans -13-dimethylcyclohexane always has one methyl group is equatorial and one methyl group axial.
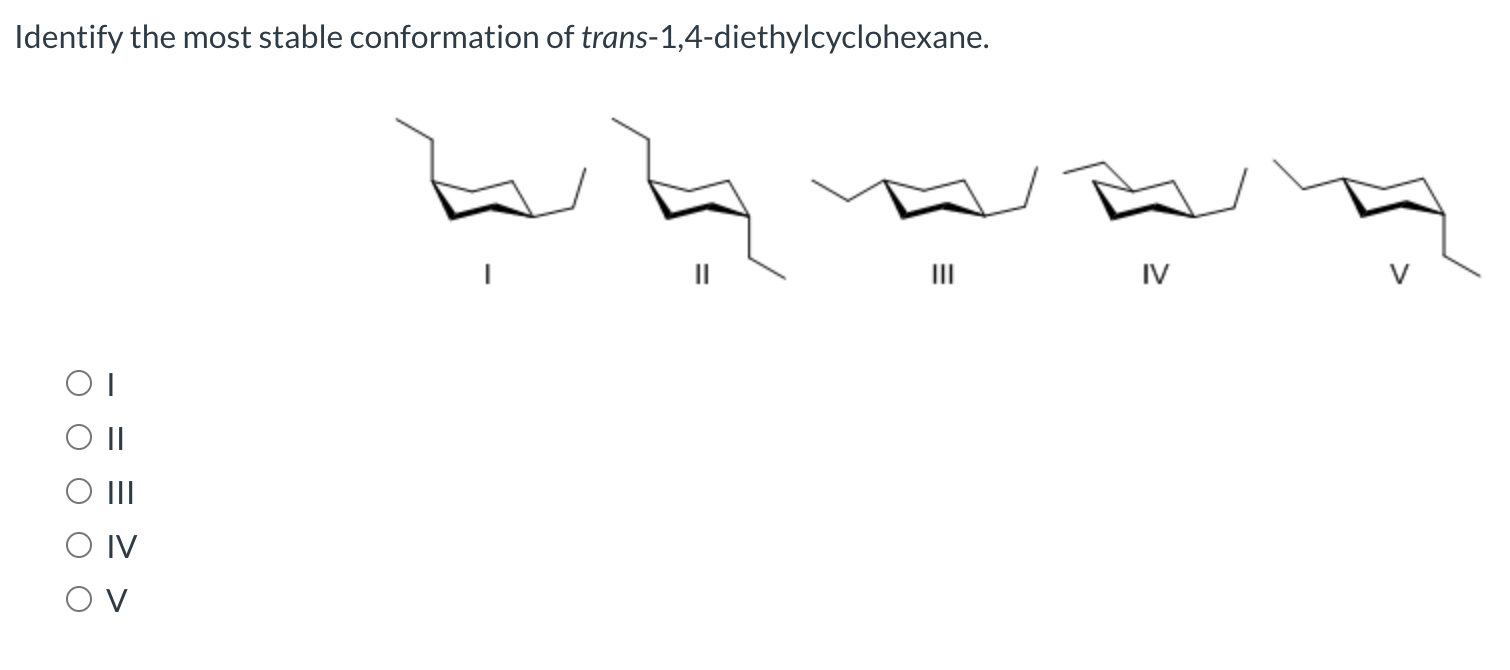
C both groups are axial in a chair conformation. The correct option is C. IV OT III OIV OV.
41 Naming Cycloalkanes 42 Cistrans Isomerism In Cycloalkanes 43 Stability Of Cycloalkanes. Find step-by-step Chemistry solutions and your answer to the following textbook question. Cis 1 4 Dimethylcyclohexane Chair - 17 images - media portfolio solved for cis 1 3 dimethylcyclohexane which two chair c for cis 1 3 dimethylcyclohexane which two chair conformations are in 1 3 dimethylcyclohexane cis trans.
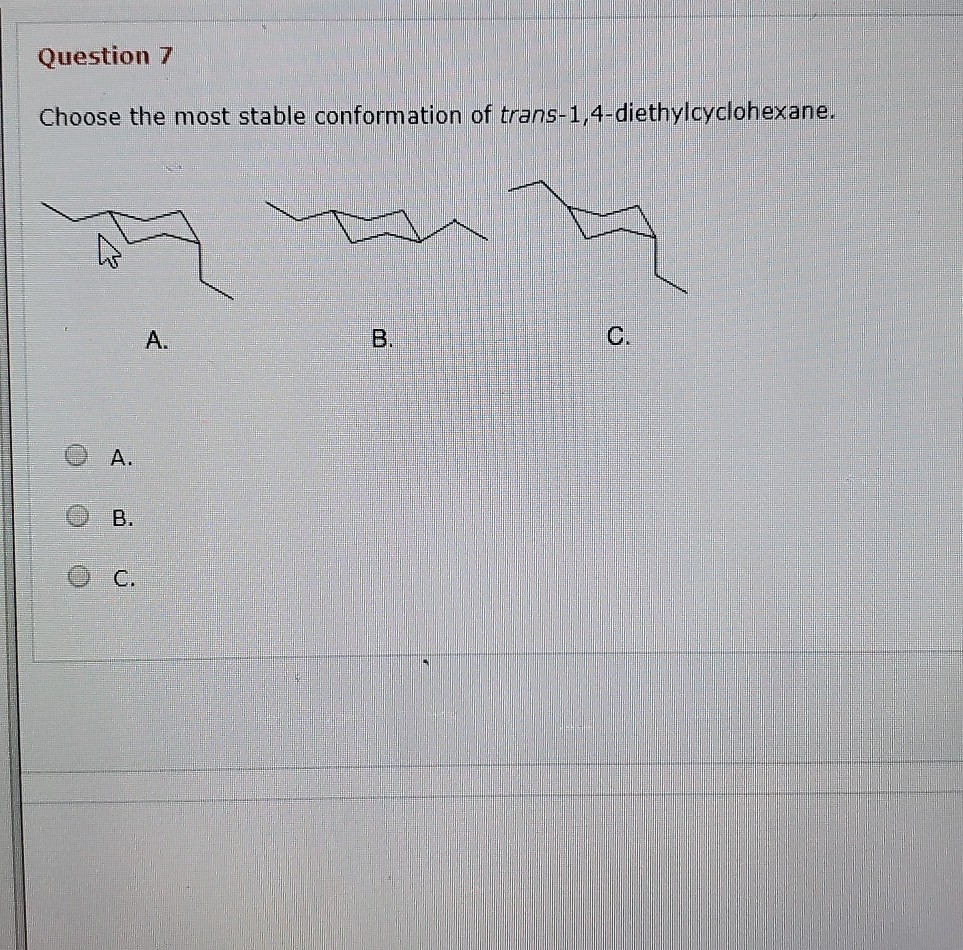
Solved Question 7 Choose The Most Stable Conformation Of Chegg Com

Solved Choose The Most Stable Chair Conformer Of Chegg Com
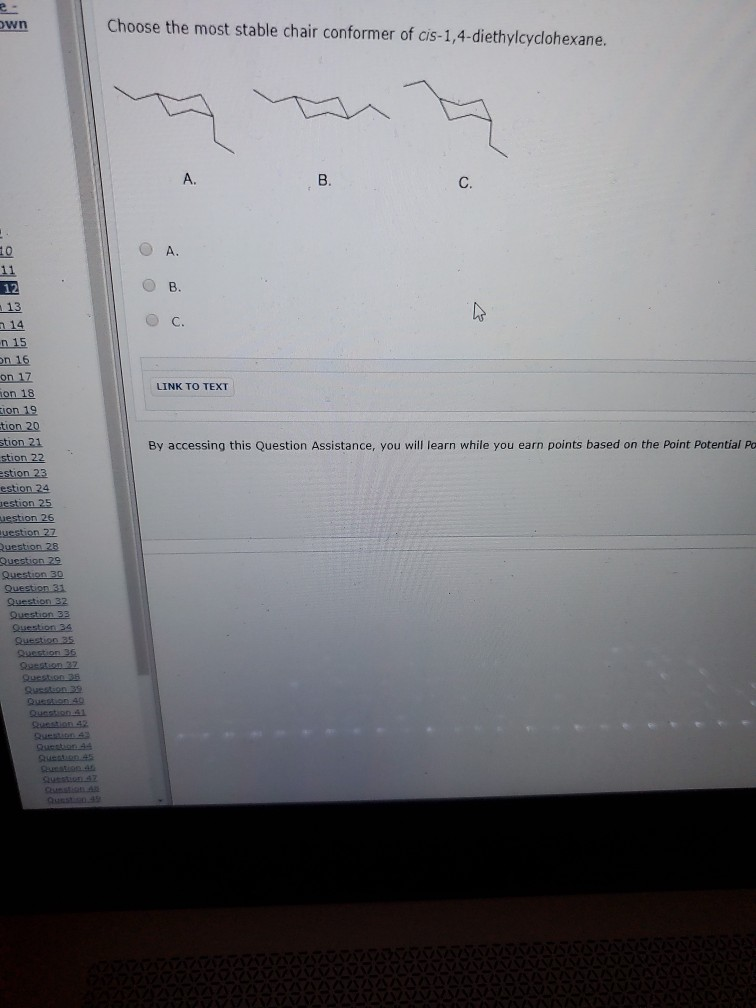
Solved Wn Choose The Most Stable Chair Conformer Of Chegg Com
Comments
Post a Comment